Pd-Catalyzed one-pot sequential cross-coupling reactions of tetrabromothiophene†
Abstract
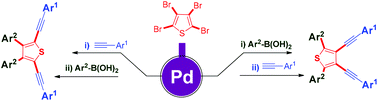
Unsymmetrical one-pot sequential cross-coupling reactions of sterically hindered tetrabromothiophene with arylboronic acid and an alkyne/alkene to afford selective bi-, tri-, and tetrasubstituted aryl/alkynyl-thiophenes with the aid of a palladium catalyst were described. The reaction proceeds via a site-selective Suzuki/Sonogashira coupling, followed by selective Sonogashira, Suzuki and Heck coupling reactions. This methodology has demonstrated an important framework for the synthesis of organic scaffolds.


 Please wait while we load your content...
Please wait while we load your content...