“Snorkelling” vs. “diving” in mixed micelles probed by means of a molecular bathymeter†
Abstract
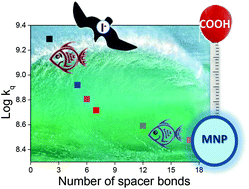
A photoactive bathymeter based on a carboxylic acid moiety covalently linked to a signalling methoxynaphthalene (MNP) fluorophore has been designed to prove the concept of “snorkelling” vs. “diving” in mixed micelles (MM). The carboxylic acid “floats” on the MM surface, while the MNP unit sinks deep in MM. The rate constants of MNP fluorescence quenching by iodide, which remains basically in water, consistently decrease with increasing spacer length, revealing different regions. This is associated with the distance MNP should “dive” in MM to achieve protection from aqueous reactants. Unequivocal proof of the exergonic photoinduced electron transfer was obtained from the UV-visible spectral signature of I3− upon steady-state photolysis. The applicability of the bathymeter was examined upon testing a family of MNP derivatives. The obtained results were validated by comparison with different lipophilicity tests: (i) a modified version of the Kow partition coefficient and (ii) the retention factor on thin layer chromatography. This concept could potentially be extended to test drugs or pharmacophores exhibiting any photoactive moiety.


 Please wait while we load your content...
Please wait while we load your content...