Formation of N-sulfonyl imines from iminoiodinanes by iodine-promoted, N-centered radical sulfonamidation of aldehydes†
Abstract
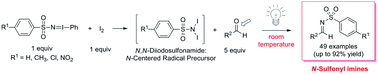
A mild and operationally convenient formation of synthetically valuable N-sulfonyl imines from a range of aryl aldehydes by reaction with iminoiodinanes (PhI![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NZ) and I2 has been developed. According to mechanistic experiments described within, the reaction is speculated to proceed through an unconventional light-promoted, N-centered radical (NCR) pathway involving a N,N-diiodosulfonamide reactive species. This method not only provides a new pathway toward the production of activated imines, but also serves as an example of a non-traditional means of carbonyl activation via an NCR species.
NZ) and I2 has been developed. According to mechanistic experiments described within, the reaction is speculated to proceed through an unconventional light-promoted, N-centered radical (NCR) pathway involving a N,N-diiodosulfonamide reactive species. This method not only provides a new pathway toward the production of activated imines, but also serves as an example of a non-traditional means of carbonyl activation via an NCR species.


 Please wait while we load your content...
Please wait while we load your content...