Iron(iii)-catalyzed chelation assisted remote C–H bond oxygenation of 8-amidoquinolines†
Abstract
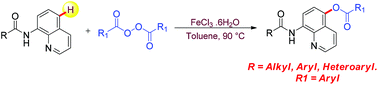
Iron catalyzed site selective and chelation assisted C–H functionalization in 8-amidoquinolines is achieved. The remote C5-benzoxylation with benzoyl peroxide produced a variety of potentially bioactive 8-arylcarboxamido-5-benzoyloxy quinoline derivatives. The efficiency of the reaction reflects from the wide substrate scope with electronic differentiation on carboxamide and acyl peroxide in addition to tolerance of halo-substitutions on either of the aryls. The reaction is additive, silver free and proceeds without the exclusion of air or moisture.


 Please wait while we load your content...
Please wait while we load your content...