Brønsted acid-catalyzed, enantioselective synthesis of 1,4-dihydroquinoline-3-carboxylates via in situ generated ortho-quinone methide imines†
Abstract
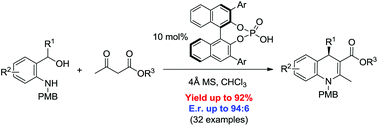
A straightforward, catalytic, enantioselective approach toward the synthesis of a broad range of 1,4-dihydroquinoline-3-carboxylates is described. Under phosphoric acid catalysis in situ-generated ortho-quinone methide imines reacted with β-keto esters to form the nitrogen heterocycles with good yields and enantioselectivities in just one synthetic step under ambient reactions conditions.


 Please wait while we load your content...
Please wait while we load your content...