Stereodivergent synthesis of right- and left-handed iminoxylitol heterodimers and monomers. Study of their impact on β-glucocerebrosidase activity†
Abstract
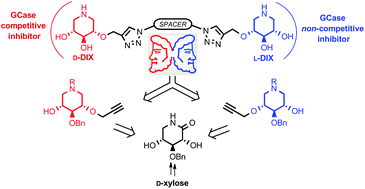
A library of dimers and heterodimers of both enantiomers of 2-O-alkylated iminoxylitol derivatives has been synthesised and evaluated on β-glucocerebrosidase (GCase), the enzyme responsible for Gaucher disease (GD). Although the objective was to target simultaneously the active site and a secondary binding site of the glucosidase, the (−)-2-iminoxylitol moiety seemed detrimental for imiglucerase inhibition and no significant enhancement was obtained in G202R, N370S and L444P fibroblasts. However, all compounds having at least one (+)-2-O-alkyl iminoxylitol are GCase inhibitors in the nano molar range and are significant GCase activity enhancers in G202R fibroblats, as confirmed by a decrease of glucosylceramide levels and by co-localization studies.


 Please wait while we load your content...
Please wait while we load your content...