Total synthesis of phenylpropanoid glycoside osmanthuside-B6 facilitated by double isomerisation of glucose–rhamnose orthoesters†
Abstract
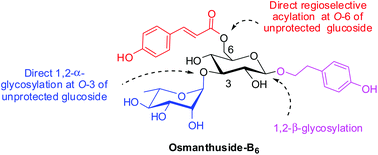
A convenient synthesis of phenylpropanoid glycoside osmanthuside-B6 is disclosed. The key steps involved regioselective coumaroylation and rhamnosylation of unprotected phenylethyl-β-D-glucopyranoside to give 2- and 3-O-rhamnosyl orthoester glucopyranosides. Rearrangement of these orthoesters followed by selective removal of their acetyl and allyl groups gave osmanthuside-B6 in 22% overall yield. The rearrangement involved a newly discovered glucose–rhamnose orthoester double isomerization process that has the potential to provide a convenient access to many complex phenylpropanoid glycosides. The synthetic route developed is envisioned to serve as a model for the preparation of phenylpropanoid glucosides having a (substituted) cinnamoyl moiety at O-6 and a saccharide moiety at O-3.


 Please wait while we load your content...
Please wait while we load your content...