Metal- and additive-free oxygen-atom transfer reaction: an efficient and chemoselective oxidation of sulfides to sulfoxides with cyclic diacyl peroxides†
Abstract
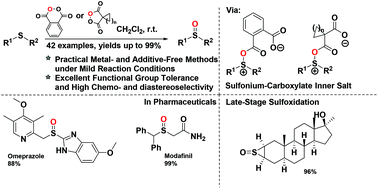
Metal- and additive-free oxidation of a series of sulfides/thioketones has been achieved using cyclic diacyl peroxides as mild oxygen sources. This protocol features simple manipulation, high chemo- and diastereoselectivity, and a broad substrate scope (up to 42 examples), tolerates many common functional groups, and is scalable and applicable to the late-stage sulfoxidation strategy. A preliminary mechanistic study by quantum mechanical calculations suggests that a single two-electron transfer process is energetically more favorable, and indicates the reactivity of cyclic diacyl peroxides distinct from conventional acyclic acyl peroxides.


 Please wait while we load your content...
Please wait while we load your content...