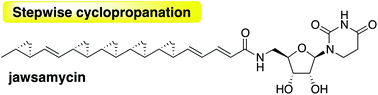
Stepwise cyclopropanation on the polycyclopropanated polyketide formation in jawsamycin biosynthesis†
Abstract
Jawsamycin is a polyketide–nucleoside hybrid with a unique polycyclopropane moiety on a single polyketide chain. The unexpected isolation of cyclopropane deficient jawsamycin analogs allowed us to propose a stepwise cyclopropanation mechanism for the enzymatic synthesis of this polyketide. The concise timing of the cyclopropanation could be regulated by a delicate balance between reaction rates of the condensation and cyclopropanation reactions.


 Please wait while we load your content...
Please wait while we load your content...