Transition-metal-free synthesis of 3-(1-pyrrolidinyl)quinolines and 3-(1-pyrrolidinyl)quinoline 1-oxides via a one-pot reaction of 3-(1-pyrrolidinyl)crotonates with nitrobenzenes†
Abstract
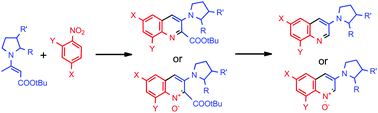
A carbanion of tert-butyl 3-(1-pyrrolidinyl)crotonate adds to nitrobenzenes to form σH-adducts, which in the presence of pivaloyl chloride and triethylamine are converted into 3-(1-pyrrolidinyl)quinolines or 3-(1-pyrrolidinyl)quinoline 1-oxides depending on the nitrobenzene structure. This is the first methodology in which a quinoline ring is constructed from a substrate bearing a pyrrolidinyl ring. Starting from optically pure enamines, the method allows synthesis of the corresponding chiral products without racemisation.


 Please wait while we load your content...
Please wait while we load your content...