Iridium catalyzed acceptor/acceptor carbene insertion into N–H bonds in water†
Abstract
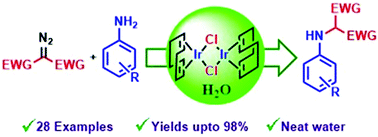
The chemistry of A and D/A carbenes (D and A are donor and acceptor groups, respectively) is known to a great extent in the literature. Nevertheless the chemistry of the A/A class of carbenes is less explored, although the A/A class of carbenes is more important in natural product synthesis. The known catalysts for A/A carbene chemistry are also less in number and are found to be efficient only in typical organic solvents. These limitations prompted us to search for new catalysts and environmentally benign reaction conditions for the A/A class of carbenes. The present study reveals that [(COD)IrCl]2 is found to be an efficient catalyst for the A/A class of carbenes, and Pd2(dba)3 for the D/A class of carbenes, for insertion into N–H bonds in water under mild reaction conditions. A reactivity comparison study with different classes of carbenes revealed that silver based catalysts are the right choice for the D/D class of carbenes for insertion into N–H bonds in water. A large number of α-amino malonates and amino esters, which are important for the synthesis of heterocycles and several natural products, have been synthesized by following the current methodology, and characterized using standard analytical and spectroscopic techniques.


 Please wait while we load your content...
Please wait while we load your content...