Redox reaction between benzyl azides and aryl azides: concerted synthesis of aryl nitriles and anilines†
Abstract
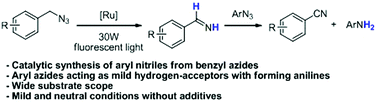
A unique and novel reaction between benzyl azides and aryl azides is described to synthesize aryl nitriles and anilines concurrently, which is catalyzed with a photoactivated diruthenium complex. N-Unsubstituted imines (N–H imines) are generated first from benzyl azides, followed by the hydrogen transfer reaction between N–H imines and aryl azides. A wide range of aryl nitriles and anilines were synthesized under neutral and mild reaction conditions.


 Please wait while we load your content...
Please wait while we load your content...