Palladium-catalyzed carbonylative synthesis of isocoumarins and phthalides by using phenyl formate as a carbon monoxide source†
Abstract
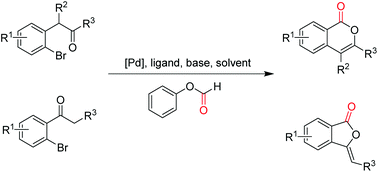
A simple and efficient palladium-catalyzed intramolecular carbonylative synthesis of isocoumarins and phthalides from the easily available starting materials by employing phenyl formate as a CO surrogate has been achieved. The approach affords target compounds in good to excellent yields with the advantages of lower toxicity, milder conditions, easy operation and wide functional group tolerance.


 Please wait while we load your content...
Please wait while we load your content...