Synthesis, SAR and biological studies of sugar amino acid-based almiramide analogues: N-methylation leads the way†
Abstract
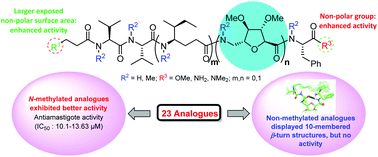
Leishmaniasis, caused by the protozoan parasites of the genus Leishmania, is one of the most neglected diseases endemic in many continents posing enormous global health threats and therefore the discovery of new antileishmanial compounds is of utmost urgency. The antileishmanial activities of a library of sugar amino acid-based linear lipopeptide analogues were examined with the aim to identify potential drug candidates to treat visceral leishmaniasis. It was found that among the synthesized analogues, most of the permethylated compounds exhibited more activity in in vitro studies against intra-macrophagic amastigotes than the non-methylated analogues. SAR and NMR studies revealed that introduction of the N-methyl groups inhibited the formation of any turn structure in these molecules, which led to their improved activities.


 Please wait while we load your content...
Please wait while we load your content...