A study of diketopiperazines as electron-donor initiators in transition metal-free haloarene–arene coupling†
Abstract
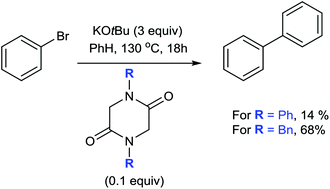
Several diketopiperazines have been shown to promote carbon–carbon coupling between benzene and aryl halides in the presence of potassium tert-butoxide and without the assistance of a transition metal catalyst. The structure of the diketopiperazine has an influence on its reductive potential and can help to promote the coupling of the more challenging aryl bromides with benzene.


 Please wait while we load your content...
Please wait while we load your content...