Facile synthesis of thietanes via ring expansion of thiiranes†
Abstract
Thietanes are pharmaceutically important cores of some biological compounds and intermediates of organic synthesis. Various thietanes were prepared from thiiranes via ring expansion through a reaction with trimethyloxosulfonium iodide in the presence of sodium hydride. The reaction process is a nucleophilic ring-opening reaction of thiiranes with dimethyloxosulfonium methylide, generated from trimethyloxosulfonium iodide and sodium hydride, and subsequent intramolecular displacement (cyclization) of thiolates to the dimethyloxosulfonium moiety. The current method provides a new strategy for efficient preparation of thietanes from readily available thiiranes.
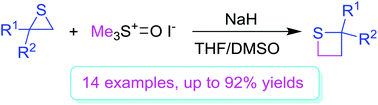

 Please wait while we load your content...
Please wait while we load your content...