Solvent effects on the folding of o-phenylene oligomers†
Abstract
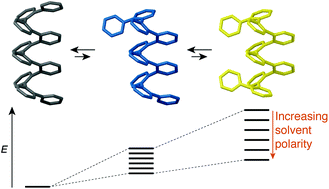
ortho-Phenylene oligomers fold into compact helical conformations in solution, and have therefore recently emerged as a class of foldamers. Previous work has shown that their folding is controlled by arene–arene stacking interactions parallel to the helical axis. Such interactions might reasonably be expected to be sensitive to solvent, but little is known of solvent effects in this system. Here, we report on the behavior of a representative set of o-phenylene oligomers in solvents ranging from non-polar (benzene) to polar and protic (methanol and water). The oligomers have been synthesized using post-oligomerization functionalization by click chemistry. Their folding is good in all solvents studied, but becomes measurably worse as the dielectric constant of the solvent increases. Thus, in contrast to the behavior of many other classes of aromatic foldamers, the folding propensity of o-phenylenes does not appear to be strongly affected by the solvophobic effect. Instead, the greater polarity of “frayed end” states governs their behavior.


 Please wait while we load your content...
Please wait while we load your content...