Boronic esters as protective groups in carbohydrate chemistry: processes for acylation, silylation and alkylation of glycoside-derived boronates†
Abstract
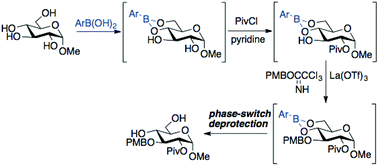
Procedures for selective installation of acyl, silyl ether and para-methoxybenzyl (PMB) ether groups to glycoside substrates have been developed, employing phenylboronic esters as protected intermediates. The sequence of boronic ester formation, functionalization and deprotection can be accomplished with only a single purification step, and the boronic acid component can be recovered and reused after deprotection. Key advances include the identification of reaction conditions for installation of PMB groups in the presence of boronic esters, and the use of the ‘phase switching’ protocol developed by Hall and co-workers as an efficient method for boronic ester cleavage. The relatively mild conditions for boronate deprotection are tolerant of several functional groups, including esters, silyl ethers, ketals and thioglycosides.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry

 Please wait while we load your content...
Please wait while we load your content...