Probing the hydrolytic reactivity of 2-difluoromethyl pyrroles†
Abstract
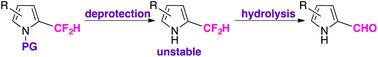
α-Difluoromethyl pyrroles were found to be stable while N-protected with an electron-withdrawing group. Due to the propensity of pyrroles to access azafulvenium-like intermediates, the C–F bonds of an α-difluoromethyl substituent are labile under hydrolytic conditions. The presence of certain electron-withdrawing substituents about the pyrrolic ring can accelerate this process, as determined through a kinetic comparison of the deprotection and subsequent hydrolysis reactions of N-protected β-aryl α-difluoromethyl pyrroles.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry

 Please wait while we load your content...
Please wait while we load your content...