A critical role of catalyst morphology in low-temperature synthesis of carbon nanotube–transition metal oxide nanocomposite†
Abstract
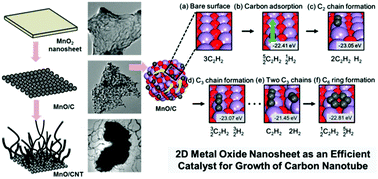
The effect of the catalyst morphology on the growth of carbon nanotubes (CNT) on nanostructured transition metal oxides was investigated to study a novel low-temperature synthetic route to functional CNT–transition metal oxide nanocomposites. Among several nanostructured manganese oxides with various morphologies and structures, only exfoliated 2D nanosheets of layered MnO2 acted as an effective catalyst for the chemical vapor deposition of CNT at low temperatures of 400–500 °C, which emphasizes the critical role of the catalyst morphology in CNT growth. Heat treatment of the MnO2 nanosheets under a C2H2 flow induced the deposition of CNT, as well as a phase transition to a 2D ordered assembly of MnO nanoparticles. The resulting CNT–MnO nanocomposites displayed excellent functionalities in Li-ion electrodes with huge discharge capacities and good rate characteristics, which highlights the usefulness of the present method for studying functional CNT–metal oxide nanocomposites. Electron microscopy and density functional theory calculations propose a formation mechanism via the efficient adsorption of carbon on the MnO2 nanosheets followed by the surface diffusion of carbon. It is of prime importance that the substitution of Fe for layered MnO2 nanosheets remarkably improved the efficiency of the formation of CNT by enhancing the surface adsorption of carbon species. This is the first report of the efficient growth of CNT at a very low temperature of 400 °C. The universal merit of the 2D nanosheet morphology was confirmed by the successful synthesis of a CNT–TiO2 nanocomposite with exfoliated titanate nanosheets. The present study demonstrates that employing exfoliated transition metal oxide nanosheets as catalysts provides an efficient low-temperature synthetic route to functional CNT–transition metal oxide nanocomposites.


 Please wait while we load your content...
Please wait while we load your content...