Nucleophile and base differentiation of pyridine with tetrahalocatechols and the formation of manganese(iii)-catecholate and pyridinium-catecholate complexes for the in situ generation of H2O2 from O2†
Abstract
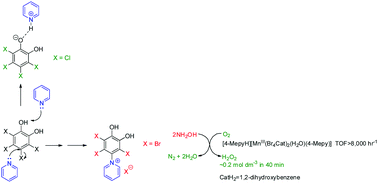
Crystal structures of two novel pyridinium catecholate compounds (1,2-dihydroxy-3,5,6-trichlorobenzene-4-pyridinium chloride and 1,2-dihydroxy-3,5,6-tribromobenzene-4-pyridinium bromide) were obtained by the reaction of pyridine with tetrachloro-o-benzoquinone (in the presence of hydroxylamine) and tetrabromocatechol respectively. A similar reaction with tetrachlorocatechol as a starting substrate showed pyridine to act as a base rather than a nucleophile, with a crystal structure of the pyridinium-catecholate salt obtained. The role of a number of manganese-catecholate complexes as catalysts in the reduction of dioxygen to hydrogen peroxide was also investigated. Diaqua-bis(3,5,6-tribromobenzene-4-pyridinium catecholate)manganese(III) bromide·MeOH, [pyH][MnIII(Br4Cat)2(H2O)(py)] and [4-MepyH][MnIII(Br4Cat)2(H2O)(4-Mepy)] (where CatH2 = catechol) were synthesised and characterised by melting point, FTIR, CHN (and Mn) analysis, mass spectrometry and UV-Vis spectroscopy. All showed catalytic behaviour in dioxygen reduction at 20 ± 1 °C and pH 8.00 in the presence of hydroxylamine as reducing substrate, with initial rates of hydrogen peroxide generation and turnover frequencies of up to 11.2 × 10−5 mol dm−3 s−1 and 8060 h−1 respectively in the presence of a 30-fold molar excess of ligand.


 Please wait while we load your content...
Please wait while we load your content...