Pd-Catalyzed Suzuki coupling reactions of aryl halides containing basic nitrogen centers with arylboronic acids in water in the absence of added base†
Abstract
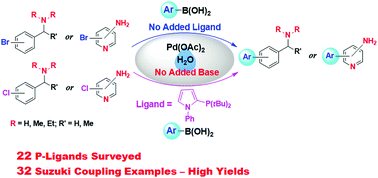
The Pd-catalyzed Suzuki coupling reactions of a series of aryl chlorides and aryl bromides containing basic nitrogen centers with arylboronic acids in water in the absence of added base are reported. The reactions proceed either partially or entirely under acidic conditions. After surveying twenty-two phosphorus ligands, high yields of products were obtained with aryl chlorides only when a bulky ligand, 2-(di-tert-butyl-phosphino)-1-phenyl-1H-pyrrole (cataCXium®PtB) was used. In contrast, aryl bromides produced high yields of products in the absence of both added base and added ligand. In order to explore the Suzuki coupling process entirely under acidic conditions, a series of reactions were conducted in buffered acidic media using several model substrates. 4-Chlorobenzylamine, in the presence of cataCXium®PtB, produced high yields of product at buffered pH 6.0; the yields dropped off precipitously at buffered pH 5.0 and lower. The fall-off in yield was attributed to the decomposition of the Pd–ligand complex due to the protonation of the ligand in the more acidic aqueous media. In contrast, in the absence of an added ligand, 4-amino-2-chloropyridine produced quantitative yields at buffered pH 3.5 and 4.5 while 4-amino-2-bromopyridine produced quantitative yields in a series of buffered media ranging from pH 4.5 to 1.5. These substrates are only partially protonated in acidic media and can behave as active Pd ligands in the Suzuki catalytic cycle.


 Please wait while we load your content...
Please wait while we load your content...