A two-pocket Schiff-base molecule as a chemosensor for Al3+†
Abstract
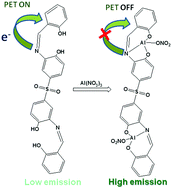
4,4′-Sulfonylbis(2-(-2-hydroxybenzylideneamino)phenol) (H4L) was synthesized by the reaction between bis-(3-amino-4-hydroxy phenyl)sulphone and salicylaldehyde (1 : 2 ratio) and has been characterized by standard methods. H4L shows a weak emission peak at 485 nm in 10 mM HEPES buffer in water : DMSO (1 : 9, v/v) (pH = 6.7) at room temperature. There is a significant enhancement in the emission intensity at 485 nm only in the presence of Al3+. Other relevant metal ions could not considerably alter its emission spectrum, indicating that H4L is a selective fluorescent probe for Al3+. H4L forms a complex with Al3+ in a 1 : 2 ratio, as is evident from Job's analysis and mass spectral data. There is a significant increase in the quantum yield and lifetime of the probe upon complex formation with aluminum. The limit of detection has been determined and shows moderate sensitivity towards the metal ion. A pH dependent study indicated that the emission intensity of H4L increases sharply when the pH of the medium is more than 7. The emission intensity of H4L in the presence of one equivalent of Al3+ starts increasing when the pH is ∼5.0. Thus, all the spectral studies were performed at pH 6.7. Some theoretical calculations were performed to investigate the spectral transitions. H4L was used for the identification of Al3+ using sea water.


 Please wait while we load your content...
Please wait while we load your content...