Multifunctionality and size of the chloranilate ligand define the topology of transition metal coordination polymers†
Abstract
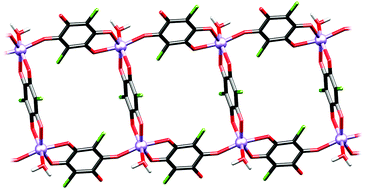
A series of eight novel complexes of chloranilic acid (CA) with the first-row transition metals (M = Mn, Cr, Ni, Co) were prepared and characterised by single-crystal X-ray diffraction, IR spectroscopy and polycrystalline X-band electron spin resonance (ESR) spectroscopy. All studied complexes revealed distorted octahedral arrangements. Three coordination modes of the chloranilate ligand are observed: bidentate, bis(bidentate) and, a novel one, mono+bidentate bridging, which can easily be distinguished by IR spectra. The size of the metal atom and different modes of chloranilate coordination affect crystal-packing topologies: 0D (mononuclear or dinuclear) and 1D (zig-zag chains or ladder-like chains). However, the ancillary ligands have a minor influence on the topology of the systems studied.


 Please wait while we load your content...
Please wait while we load your content...