A bio-inspired zinc finger analogue anchored in 2D hexagonal mesoporous silica for room temperature CO2 activation via a hydrogenocarbonate route†
Abstract
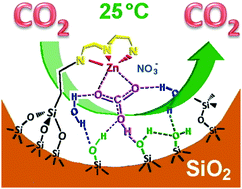
Bio-inspired diethylenetriamine–zinc(II) complexes were anchored into the nanopores of hexagonal mesoporous MCM41-like silicas targeting a carbamate free and low temperature CO2 recycling process. A step-by-step approach was adopted to perform an in situ synthesis in order to mimic the zinc finger of carbonic anhydrases, the fastest family of enzymes. In the presence of a surface-masking pattern of TMA+ ions, some silanol groups were capped using grafted trimethylsilyl functions, TMSgr, (gr for grafted). After removing the masking ions, a tridentate diethylenetriamine ligand was anchored using diethylenetriamine propyltrimethoxysilane. The so-called DETAan ligands (an for anchored) were partially monoprotonated using either cyclohexane or isopropanol as a solvent. Nonetheless, up to two thirds of them were metallated by Zn(II) ions, leading to the targeted anchored zinc finger mimic [Zn(DETAan)L]+(L = Cl− or OH−). CO2 is then adsorbed at room temperature and in humid ambient air by the formation of an intermediate hydrogenocarbonate–zinc complex. Specific IR signatures at 1330 and 1400 cm−1 together with characteristic C 1s and Zn 2p3/2 XPS binding energies at 286.4 and 1024.6 eV advocate for a rather symmetrical bidentate [η2-CO3] structural unit in the anchored complex [Zn(DETAan)(η2-HCO3*)]+, where the Zn(II) ion is most likely pentacoordinated. The internal pH value varied by less than 0.5 depending on the metal reacting with the DETAan ligand and its ability to generate HCO3−, due to the buffering effect of surface silanol and amino groups according to the level of protonation of the DETA moieties measured from the N 1s XPS spectra. In contrast to nitrate ions, chloride ions were found to inhibit the formation of hydrogenocarbonate.


 Please wait while we load your content...
Please wait while we load your content...