Design, synthesis and structure–activity relationships of (±)-isochaihulactone derivatives
Abstract
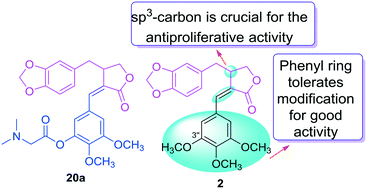
Z-K8 (2), the racemic form of isochaihulactone (1), previously showed significant antitumor effects in A549 and LNCaP tumor-bearing mice. In the present study, 17 derivatives of 2 were designed, synthesized and evaluated for their anti-proliferative activity against four human tumor cell lines. All new derivatives exhibited high potency against A549 and P-glycoprotein (P-gp)-overexpressing KB-VIN. One of our new derivatives exhibited greater activity against three tested tumor cells (A549, KB, and KB-VIN) than 2, and induced cell cycle arrest in the G2/M phase. Moreover, SAR conclusions were first established for this series of compounds. Our study clearly identified a structural feature that should be retained for good activity and also a moiety that can tolerate various modifications and, thus, is ideal for further changes.


 Please wait while we load your content...
Please wait while we load your content...