Orthoester functionalized N-guanidino derivatives of 1,5-dideoxy-1,5-imino-d-xylitol as pH-responsive inhibitors of β-glucocerebrosidase†
Abstract
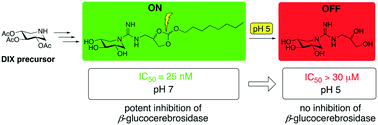
Alkylated guanidino derivatives of 1,5-dideoxy-1,5-imino-D-xylitol bearing an orthoester moiety were prepared using a concise synthetic protocol. Inhibition assays with a panel of glycosidases revealed that one of the compounds prepared displays potent inhibition against human β-glucocerebrosidase (GBA) at pH 7.0 with IC50 values in the low nanomolar range. Notably, a significant drop in inhibitory activity is observed when the same compound is tested at pH 5.2. This pH sensitive activity is due to degradation of the orthoester functionality at lower pH accompanied by loss of the alkyl group. This approach provides a degree of control in tuning enzyme inhibition based on the local pH. Compounds like those here described may serve as tools for studying various lysosomal storage disorders such as Gaucher disease. In this regard, the most active compound was also evaluated as a potential pharmacological chaperone by assessing its effect on GBA activity in an assay employing fibroblasts from Gaucher patients.


 Please wait while we load your content...
Please wait while we load your content...