Synthesis of potential bisphenol A substitutes by isomerising metathesis of renewable raw materials†
Abstract
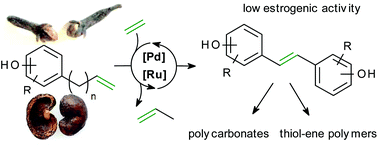
Isomerising metathesis is introduced as a sustainable method to produce dihydroxystilbene derivatives from eugenol, a clove oil ingredient, and cardanol from cashew nut shell liquid. Hydrogenation of the dihydroxystilbenes provided their di(hydroxyphenyl)ethane analogues. Initial studies to convert these monomers into polycarbonates and thiol–ene polymers support their potential to replace the petrol-derived bisphenol A (BPA). The estrogenic activity of the monomers derived from cardanol was found to be in the same range as that of BPA, a known endocrine disruptor. In contrast, eugenol-derived materials were found to be non-estrogenic, opening up new perspectives for bio-based food packaging materials.


 Please wait while we load your content...
Please wait while we load your content...