cis,cis-Muconic acid isomerization and catalytic conversion to biobased cyclic-C6-1,4-diacid monomers†
Abstract
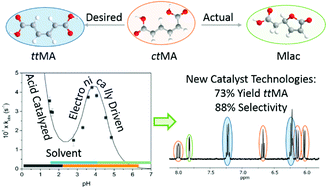
Renewable terephthalic and 1,4-cyclohexanedicarboxylic acids can be produced from biomass via muconic acid using a combination of biological and chemical processes. In this conversion scheme, cis,cis-mucononic acid is first obtained by fermentation using either sugar or lignin monomers as a feedstock. The diunsaturated cis,cis-diacid is then isomerized to trans,trans-muconic acid, reacted with biobased ethylene through Diels–Alder cycloaddition, and further hydrogenated or dehydrogenated to yield the desired 100% renewable cyclic dicarboxylic acid. The isomerization of cis,cis- to trans,trans-muconic acid represents the main bottleneck in this process due to undesired side reactions that promote ring closing to form lactones. Therefore, new technologies for the selective isomerization of muconic acid are urgently needed. Here, we studied the corresponding reaction kinetics to elucidate the mechanisms involved in both the isomerization and cyclization reactions with the objective to identify conditions that favor the selective formation of trans,trans-muconic acid. We demonstrate that the reactivity of muconic acid in aqueous media strongly depends on pH. Under alkaline conditions, cis,cis-muconic acid is deprotonated to the corresponding muconate dianion. This species is stable for extended periods of time and does not isomerize. Conversely, cis,cis-muconic acid readily isomerizes to its cis,trans-isomer under acidic conditions. Prolonged heating further triggers the intramolecular cyclizations through reaction of the carboxylic acid and alkene functionalities. The formation of the muconolactone and its dilactone is kinetically favored over the isomerization to trans,trans-muconic acid over a broad range of conditions. However, strategies involving the chelation of the carboxylates with inorganic salts or their solvation using polar aprotic solvents were found to hamper the ring closing reactions and allow the isomerization to trans,trans-muconic acid to proceed with high selectivity (88%). The obtained compound was further reacted with ethylene and hydrogenated to 1,4-cyclohexanedicarboxylic acid, an important monomer for the polyester and polyamide industries.


 Please wait while we load your content...
Please wait while we load your content...