Magnetically separable sulfated zirconia as highly active acidic catalysts for selective synthesis of ethyl levulinate from furfuryl alcohol†
Abstract
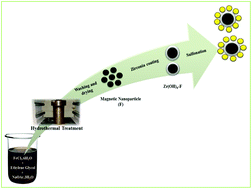
Magnetically separable sulfated zirconia catalysts were prepared by a two-step approach. Coating of zirconia around the particles helps to increase the number of sites needed for sulfate ion loading and hence enhances the acidity of the catalyst. Different molar concentrations of chlorosulfonic acid were used for sulfonation. The prepared catalysts were used for selective synthesis of ethyl levulinate using renewable substrates: furfuryl alcohol and ethanol. Ethyl levulinate has many applications in different industries including as a potential blending component in biodiesel. The catalyst could be easily separated by the use of a magnet. The influence of different parameters was investigated to reach the optimum yield of ethyl levulinate. Detailed kinetics were established for scaling up purposes. The catalyst is robust and reusable.


 Please wait while we load your content...
Please wait while we load your content...