Synthesis and characterization of uranium(iv) tetrachloro complexes in bis-pyrazolylpyridine ligand environments†‡
Abstract
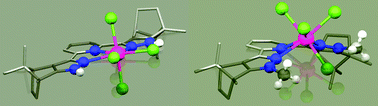
Starting from dimethyl 2,6-pyridinedicarboxylate (3), four pyridine-bridged bispyrazole ligands 1a–1d were generated in a two- or three-step synthesis sequence and further treated with UCl4 to yield the corresponding novel mononuclear uranium(IV) complexes [UIV(R′′′2L)(Cl)4] (2a–2d). Compounds 2a–2d were characterized by a variety of spectroscopic and physical methods (e.g. UV/Vis, SQUID, CV, etc.), corroborating the +4 oxidation state in 2a–2d. Single-crystal X-ray structure analyses revealed that 2a·2THF crystallizes in the orthorhombic space group Pbca, 2b·0.8THF·0.2Et2O in the monoclinic Sohncke space group P21, 2c·0.25Et2O in the monoclinic one P21/c, and finally 2d·0.5THF in the orthorhombic Sohncke space group P21212. In the solid state, complexes 2a–2d possess a distorted pentagonal–bipyramidal coordination sphere at the UIV centers and an out-of-plane shift (doop) of up to 1.12 Å, which can be explained by an increased steric pressure on the metal ions at the binding sites of the chelating ligands 1c and 1d. Finally, by combination of different 1D and 2D NMR experiments, the 1H and 13C resonances can be unequivocally assigned in the corresponding paramagnetic NMR spectra of 2a–2d.


 Please wait while we load your content...
Please wait while we load your content...