Desolvation and aggregation of sterically demanding alkali metal diarylphosphides†
Abstract
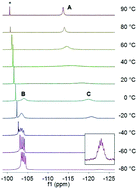
The reaction between (Dipp)2PH and one equivalent of n-BuLi, PhCH2Na or PhCH2K in THF gives the complexes [(Dipp)2P]Li(THF)3 (2a), {[(Dipp)2P]Na(THF)2}2 (3a) and [(Dipp)2P]K(THF)4 (4a), respectively [Dipp = 2,6-iPr2C6H3]. Exposure of these compounds to vacuum yields the alternative solvates [(Dipp)2P]Li(THF)2 (2b), [(Dipp)2P]Na(THF)1.5 (3b), and [(Dipp)2P]K (4b), respectively; the alternative adduct [(Dipp)2P]Na(PMDETA) (3c) was prepared by treatment of 3a with PMDETA. Treatment of (Dipp)(Mes)PH or (Mes)2PH with one equivalent of n-BuLi in THF gives the complexes [(Dipp)(Mes)P]Li(THF)3 (7a) and [(Mes)2P]2Li2(THF)2(OEt2) (8a) after crystallisation from diethyl ether [Mes = 2,4,6-Me3C6H2]; crystallisation of 8a from hexane gives the alternative adduct [(Mes)2P]Li(THF)3 (8b). Exposure of 7a, 8a and 8b to vacuum leads to loss of coordinated solvent, yielding the solvates [(Dipp)(Mes)P]Li(THF)2 (7b) and [(Mes)2P]Li(THF) (8c). The solid-state structures of complexes 2a, 3a, 3c, 4a, 7a, 8a, and 8b have been determined by X-ray crystallography. Variable-temperature 31P{1H} and 7Li NMR spectroscopy indicates that 2b, 3b and 7b are subject to a monomer–dimer equilibrium in solution, where the monomeric forms are favoured at low temperature. In contrast, variable-temperature 31P{1H} and 7Li NMR spectroscopy suggests that 8c is subject to a dynamic equilibrium between a dimer and a cyclic trimer in solution, where the trimer is favoured at low temperatures.


 Please wait while we load your content...
Please wait while we load your content...