Towards the upgrading of fermentation broths to advanced biofuels: a water tolerant catalyst for the conversion of ethanol to isobutanol†
Abstract
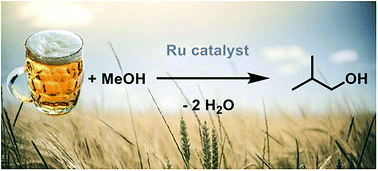
Isobutanol is an ideal gasoline replacement due to its high energy density, suitable octane number and compatibility with current engine technology. It can be formed by the Guerbet reaction in which (bio)ethanol and methanol mixtures are converted to this higher alcohol in the presence of a suitable catalyst under basic conditions. A possible limitation of this process is the catalyst's water tolerance; a twofold problem given that water is produced as a by-product of the Guerbet reaction but also due to the need to use anhydrous alcoholic feedstocks, which contributes significantly to the cost of advanced biofuel production. Isobutanol formation with pre-catalyst trans-[RuCl2(dppm)2] (1) has been shown to be tolerant to the addition of water to the system, achieving an isobutanol yield of 36% at 78% selectivity with water concentrations typical of that of a crude fermentation broth. Key to this success is both the catalyst's tolerance to water itself and the use of a hydroxide rather than an alkoxide base; other catalysts explored are less effective with hydroxides. Alcoholic drinks have also been used as surrogates for the fermentation broth: the use of lager as the ethanol source yielded 29% isobutanol at 85% selectivity in the liquid phase.


 Please wait while we load your content...
Please wait while we load your content...