Double salt ionic liquids based on 1-ethyl-3-methylimidazolium acetate and hydroxyl-functionalized ammonium acetates: strong effects of weak interactions†
Abstract
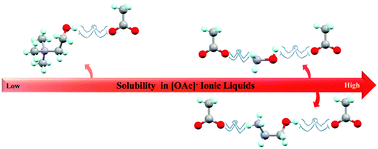
The properties of double salt ionic liquids based on solutions of cholinium acetate ([Ch][OAc]), ethanolammonium acetate ([NH3(CH2)2OH][OAc]), hydroxylammonium acetate ([NH3OH][OAc]), ethylammonium acetate ([NH3CH2CH3][OAc]), and tetramethylammonium acetate ([N(CH3)4][OAc]) in 1-ethyl-3-methylimidazolium acetate ([C2mim][OAc]) were investigated by NMR spectroscopy and X-ray crystallography. Through mixture preparation, the solubility of [N(CH3)4][OAc] is the lowest, and [Ch][OAc] shows a 3-fold lower solubility than the other hydroxylated ammonium acetate-based salts in [C2mim][OAc] at room temperature. NMR and X-ray crystallographic studies of the pure salts suggest that the molecular-level mechanisms governing such miscibility differences are related to the weaker interactions between the –NH3 groups and [OAc]−, even though three of these salts possess the same strong 1 : 1 hydrogen bonds between the cation –OH group and the [OAc]− ion. The formation of polyionic clusters between the anion and those cations with unsatisfied hydrogen bond donors seems to be a new tool by which the solubility of these salts in [C2mim][OAc] can be controlled.


 Please wait while we load your content...
Please wait while we load your content...