A study on an unusual SN2 mechanism in the methylation of benzyne through nickel-complexation†
Abstract
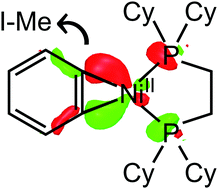
In this study, three reaction mechanisms of a benzyne–nickel (Ni) complex ([Ni(C6H4)(dcpe)]) with iodomethane during the methylation process were investigated, namely (a) SN2 reaction of the benzyne–Ni complex with iodomethane, (b) concerted σ-bond metathesis during the bond breaking/forming processes, and (c) oxidative addition of iodomethane to the Ni-center and the subsequent reductive elimination process. DFT calculations revealed that the reaction barrier of the SN2 reaction is slightly lower than those of the other mechanisms. The results of orbital analyses suggest that [Ni(C6H4)(dcpe)] forms a metallacycle structure between benzyne and the NiII (3d8) center instead of the η2-structure with the Ni0 (3d10) center. The metallacycle structures became inappropriate as the intermediates of oxidative addition in the formation of the NiII–Me bond, avoiding further oxidation to the high-valent NiIV. The high free energy along σ-bond metathesis was generated from the steric hindrance, thus invoking methylation and Ni–I bond formation concertedly.


 Please wait while we load your content...
Please wait while we load your content...