Confined water dynamics in a hydrated photosynthetic pigment–protein complex
Abstract
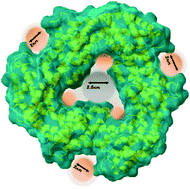
Water is of fundamental importance for life. It plays a critical role in all biological systems. In phycocyanin, a pigment–protein complex, the hydration level influences its absorption spectrum. However, there is currently a gap in the understanding of how protein interfaces affect water's structure and properties. This work presents combined dielectric and calorimetric measurements of hydrated phycocyanin with different levels of hydration in a broad temperature interval. Based on the dielectric and calorimetric tests, it was shown that two types of water exist in the phycocyanin hydration shell. One is confined water localized inside the phycocyanin ring and the second is the water that is embedded in the protein structure and participates in the protein solvation. The water confined in the phycocyanin ring melts at the temperature 195 ± 3 K and plays a role in the solvation at higher temperatures. Moreover, the dynamics of all types of water was found to be effected by the presence of the ionic buffer.


 Please wait while we load your content...
Please wait while we load your content...