Plasmonic support-mediated activation of 1 nm platinum clusters for catalysis†
Abstract
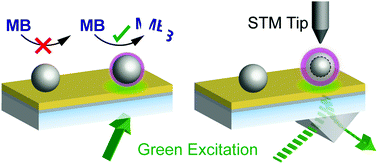
Nanometer-sized metal clusters are prime candidates for photoactivated catalysis, based on their unique tunable optical and electronic properties, combined with a large surface-to-volume ratio. Due to the very small optical cross sections of such nanoclusters, support-mediated plasmonic activation could potentially make activation more efficient. Our support is a semi-transparent gold film, optimized to work in a back-illumination geometry. It has a surface plasmon resonance excitable in the 510–540 nm wavelength range. Ptn clusters (size distribution peaked at n = 46 atoms) have been deposited onto this support and investigated for photoactivated catalytic performance in the oxidative decomposition of methylene blue. The Pt cluster catalytic activity under illumination exceeds that of the gold support by more than an order of magnitude per active surface area. To further investigate the underlying mechanism of plasmon-induced catalysis, the clusters have been imaged with optically-assisted scanning tunneling microscopy under illumination. The photoactivation of the Pt clusters via plasmonic excitation of the support and subsequential electronic excitation of the clusters can be imaged with nanometer resolution. The light-induced tunneling current on the clusters is enhanced relative to the gold film support.


 Please wait while we load your content...
Please wait while we load your content...