The long-range convergence of the energetic properties of the water monomer in bulk water at room temperature†
Abstract
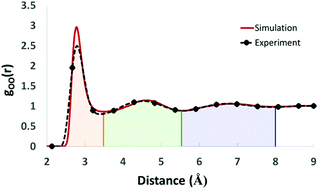
The Interacting Quantum Atoms (IQA) energy partitioning scheme has been applied to a set of liquid water largely spherical clusters (henceforth called spheres) of up to 9 Å radius, with a maximum cluster size of 113 molecules. This constitutes half of the commonly used 216 molecules in a typical simulation box of a liquid water box, and to our knowledge is the largest analysis of this kind ever undertaken. As well as demonstrating the topological analysis of large systems, which has only recently become computationally feasible, important long range properties of liquid water are obtained. The full topological partitioning of each sphere into atomic basins is used to consider the long-range convergence of the energetic and multipolar properties of the water molecule at the centre of each sphere. It is found that the total molecular energy converges to its 9 Å value after 7 Å, which corresponds to approximately the first three solvation shells, while the molecular dipole and quadrupole moments approximately converge after 5.5 Å, which corresponds to approximately the first two solvation shells. The effect of water molecule flexibility is also considered.


 Please wait while we load your content...
Please wait while we load your content...