A study on the progress of mutarotation above and below the Tg and the relationship between constant rates and structural relaxation times
Abstract
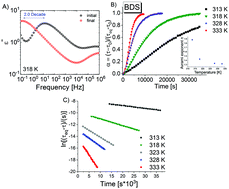
Comprehensive FTIR studies on the progress of mutarotation in D-fructose mixed with maltitol have been carried out over a wide range of temperatures, both above and below the glass transition temperature Tg. In addition to the analysis of single bands, we have developed a completely new approach considering the full spectral range to follow the overall progress of the reaction. We have found that at the calorimetric Tg, there is a clear change in the temperature dependence of constant rates. The activation barrier for mutarotation changes from around 59 kJ mol−1 (the supercooled state) to around 249 kJ mol−1 (the glassy state). This dramatic variation in the activation barrier is consistent with the change in the mechanism of this specific chemical conversion, as theoretically considered by Wlodarczyk et al. [Phys. Chem. Chem. Phys., 2014, 16, 4694–4698]. Alternatively, it can also be connected to the change in the viscosity of the sample. Additionally, we investigated the relationship between constant rates (k) of mutarotation, structural relaxation times (τα), and dc conductivity (σdc) above and below the glass transition temperature. It was found that there was a linear correlation between all these quantities; they scale with various exponents changing at Tg. Our results also indicate that a single activation barrier might not be sufficient to describe the mutarotation process.


 Please wait while we load your content...
Please wait while we load your content...