The role of ion–water interactions in determining the Soret coefficient of LiCl aqueous solutions†
Abstract
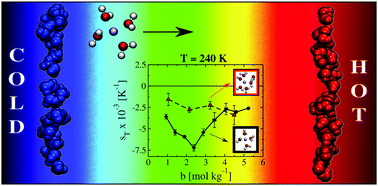
The application of a thermal gradient to an aqueous electrolyte solution induces the Soret effect, and the salt migrates towards hot (thermophilic) or cold regions (thermophobic). Experimental studies of LiCl reported changes in the sign of the Soret coefficient as well as a minimum in this coefficient at specific salt concentrations and temperatures. At the minimum the thermodiffusive response of the solution is enhanced significantly. We have performed non-equilibrium molecular dynamics simulations of LiCl solutions to quantify the dependence of the sign change and minimum of the Soret coefficient with salt concentration and temperature. We find that the ion mass plays a secondary role in determining the magnitude of the Soret coefficient, while the diameter of the cation has a significant impact on the coefficient and on the observation of the minimum. Our simulations show that the ordering of water around Li+ plays a key role in determining the Soret coefficient of LiCl salts.


 Please wait while we load your content...
Please wait while we load your content...