Spontaneous NaCl-doped ice at seawater conditions: focus on the mechanisms of ion inclusion†
Abstract
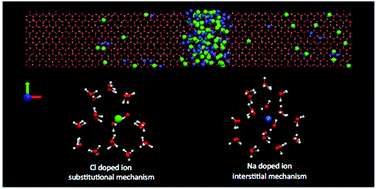
Molecular dynamics simulations on the microsecond time scale have been performed on an aqueous solution of TIP4P/2005 water and NaCl by using the direct coexistence technique to study the ice growth and the ice/liquid interface water. At ambient pressure, for temperatures above the eutectic point of the salt and at seawater concentrations the brine rejection phenomenon and the spontaneous growth of an ice slab doped by the salt are obtained, as found in natural terrestrial and planetary environments. Experiments indicate that Cl− goes via substitution to ice sites. In line with this evidence we find a new result: the Cl− ion included in the lattice always substitutes not one but two water molecules, leaving the surrounding ice structure not distorted. The Na+ ion shows a lower probability of being included in the ice and it occupies an interstitial site, causing a local distortion of the lattice. No signs of significant ion diffusion are observed in the lattice.


 Please wait while we load your content...
Please wait while we load your content...