Reaction monitoring reveals poisoning mechanism of Pd2(dba)3 and guides catalyst selection†
Abstract
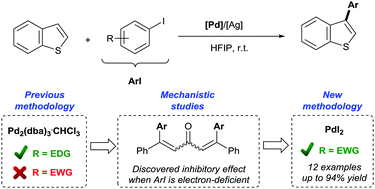
We have discovered that the dba ligand in the commonly used Pd2(dba)3·CHCl3 cross-coupling pre-catalyst is susceptible to bis-arylation when used in the presence of aryl iodides. The in situ formed dbaAr2 ligands result in Pd-species with altered catalytic activity. In the case of study, the room temperature C3 arylation of benzo[b]thiophenes with aryl iodides, we have observed a marked catalyst deactivation when dba is arylated with electron-deficient aryl iodides, accounting for the poor yields obtained in the C3 arylation reactions with these aryl iodides. Based on these studies, we report a new catalytic system, employing a dba-free Pd catalyst, which allows for the first time the direct C3 arylation of benzo[b]thiophenes with electron-deficient aryl iodides at room temperature.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...