Fluorescent chirality recognition by simple boronate ensembles with aggregation-induced emission capability†
Abstract
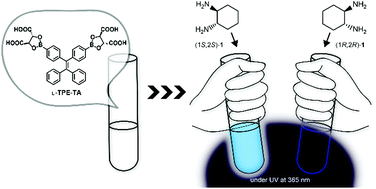
Chiral ensembles were spontaneously formed in solution through boronate esterification of structurally defined di(boronic acid)-appended tetraphenylethylene (DB-TPE) and commercially available L- or D-tartaric acid, showing enantioselective aggregation behavior for chiral diamines as well as cinchona alkaloids enabling the fluorescent recognition of their chirality.
- This article is part of the themed collection: Chemosensors and Molecular Logic


 Please wait while we load your content...
Please wait while we load your content...