Adsorption of perfluorocarboxylic acids at the silica surface†
Abstract
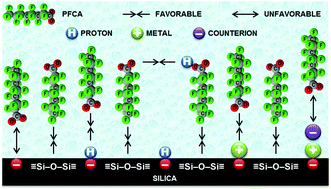
Water molecules may force the hydrophobic perfluoroalkyl chain of perfluorocarboxylic acids (PFCAs) to aggregate at hydrophobic siloxane patches (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–Si
Si–O–Si![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) ) of the silica surface despite electrostatic repulsion between surface silanol (
) of the silica surface despite electrostatic repulsion between surface silanol (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO−) and the carboxylate (–COO−) headgroup of PFCA. Acidic pH and background cations promote the adsorption further by reducing the anion–anion Coulomb repulsion.
SiO−) and the carboxylate (–COO−) headgroup of PFCA. Acidic pH and background cations promote the adsorption further by reducing the anion–anion Coulomb repulsion.


 Please wait while we load your content...
Please wait while we load your content...