Easy access to triazolinedione-endcapped peptides for chemical ligation†
Abstract
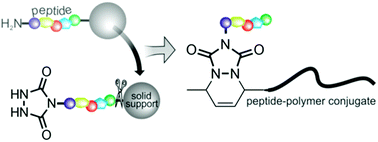
An efficient and easy route towards triazolinedione (TAD) endcapped peptides is described, in which a TAD-precursor was coupled to N-terminal amines on a solid support. Modified peptides readily reacted with diene end-functionalized poly(ε-caprolactone) of different molecular weights. The ligation proved to be orthogonal to a variety of functional groups present in natural amino acids.


 Please wait while we load your content...
Please wait while we load your content...