Development and validation of green chromatography for the determination of anthocyanins in haskap berry, mulberry and blackberry†
Abstract
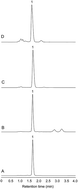
This study was aimed at developing and validating a green HPLC-DAD method to determine anthocyanins using ethanol and an alpha-hydroxy acid aqueous solution as a mobile phase. The effects of alpha-hydroxy acids on the chromatographic behaviors of cyanidin-3-O-glucoside, the most predominant anthocyanin in nature, in a C18 column were investigated. Among seven selected alpha-hydroxy acids, tartaric acid exhibited potential for improving the chromatographic separation of cyanidin-3-O-glucoside. Cyanidin-3-O-glucoside was the main anthocyanin in haskap berry, mulberry, and blackberry, and the three berries were selected for further method development and validation. The optimized mobile phase was a mixture of ethanol and a 0.25 mol L−1 tartaric acid aqueous solution at the 20 : 80 (v/v) ratio. The retention time of cyanidin-3-O-glucoside was less than 1.8 min, and an optimum separation of anthocyanins was achieved in 4 min. The developed method was validated in terms of linearity, limits of detection and quantification, precision, repeatability, stability, and recovery. The calibration curve revealed good linearity (R2 = 0.9999), and the limits of detection and quantification were 0.038 mg L−1 and 0.125 mg L−1, respectively. The relative standard deviations of intra- and inter-day precision, repeatability, and stability were less than 5%. The recoveries of cyanidin-3-O-glucoside from the berry samples were between 99% and 101%, a satisfactory level. Additionally, the optimized mobile phase had a negligible effect on the C18 column stability. Therefore, the developed method was safe, rapid, and valid for anthocyanin analysis.


 Please wait while we load your content...
Please wait while we load your content...