Heterogeneous catalase-like activity of gold(i)–cobalt(iii) metallosupramolecular ionic crystals†
Abstract
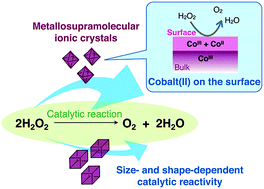
Unique heterogeneous catalase-like activity was observed for metallosupramolecular ionic crystals [AuI4CoIII2(dppe)2(D-pen)4]Xn ([1]Xn; dppe = 1,2-bis(diphenylphosphino)ethane; D-pen = D-penicillaminate; Xn = (Cl−)2, (ClO4−)2, (NO3−)2 or SO42−) consisting of AuI4CoIII2 complex cations, [1]2+, and inorganic anions, X− or X2−. Treatment of the ionic crystals with an aqueous H2O2 solution led to considerable O2 evolution with a high turnover frequency of 1.4 × 105 h−1 for the heterogeneous cobalt complexes, which was dependent on their size and shape as well as the arrangement of cationic and anionic species. These dependencies were rationalized by the presence of cobalt(II) centers on the crystal surface and their efficient exposure on the (111) plane rather than the (100) plane based on morphological and theoretical studies.


 Please wait while we load your content...
Please wait while we load your content...