Biotransformation of dehydroepiandrosterone (DHEA) by environmental strains of filamentous fungi†
Abstract
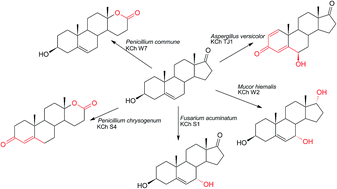
Microbial transformations of steroids are an important method of obtaining new steroid derivatives of potential pharmaceutical activity, which follows the principles of green chemistry. We studied the ability of selected filamentous fungus species to transform dehydroepiandrosterone (DHEA) and interesting DHEA derivatives were obtained. Twenty-five strains of filamentous fungi were isolated from soil and air in Wroclaw and they were used as biocatalysts. Strains of the genus Penicillium transformed the substrate into the products of Baeyer–Villiger lactonization in the D ring. Biotransformation of DHEA by Fusarium acuminatum KCh S1 strain led to a stereoselective hydroxylation at 7α (the product was obtained with 97% conversion). Androst-5-ene-3β,7α,17α-triol, compound with high anticancer activity towards glioblastoma and lymphoma cell lines was obtained with 65% yield in the culture of Mucor hiemalis KCh W2 strain. In the culture of Aspergillus versicolor KCh TJ1 strain, DHEA was transformed into androst-1,4-diene-3,17-dione (ADD) with high isolated yield.


 Please wait while we load your content...
Please wait while we load your content...