Total synthesis and confirmation of the revised structures of jiangrines A, C and D†
Abstract
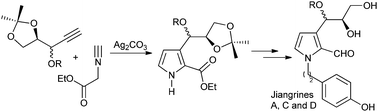
Our previous total synthesis of the proposed structures of jiangrines C and D shows that the characteristic data of synthetic samples did not match those of the natural ones, prompting us to revise their structures. Accordingly, we now accomplished the total synthesis of jiangrines A, C and D, which confirms our deduction that their molecular skeletons should compose of 2,3-disubstituted pyrrole instead of 2,5-disubstituted pyrrole. Our current synthesis features a silver-catalyzed [3 + 2] cycloaddition between a terminal alkyne and isocyanide, and was completed concisely in only seven linear steps.

 Please wait while we load your content...
Please wait while we load your content...