Pseudopeptidic compounds for the generation of dynamic combinatorial libraries of chemically diverse macrocycles in aqueous media†
Abstract
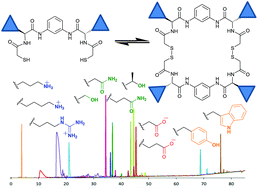
A straightforward four-step synthesis leads to the preparation of C2-symmetric dithiols containing a central aromatic core and amino acid side chains. These building blocks allow the preparation of dynamic covalent libraries of pseudopeptidic macrocycles in aqueous media that cover a broad range of polarities, functional groups and bulkiness mirroring the diversity found in natural peptides. The versatility of the generated dynamic libraries has been illustrated by the amplification of two different members from the same library upon the action of two biologically relevant templates.
- This article is part of the themed collection: Macrocycles with bio-related applications


 Please wait while we load your content...
Please wait while we load your content...